
Chemistry, 08.05.2021 01:00 mckenziealexander
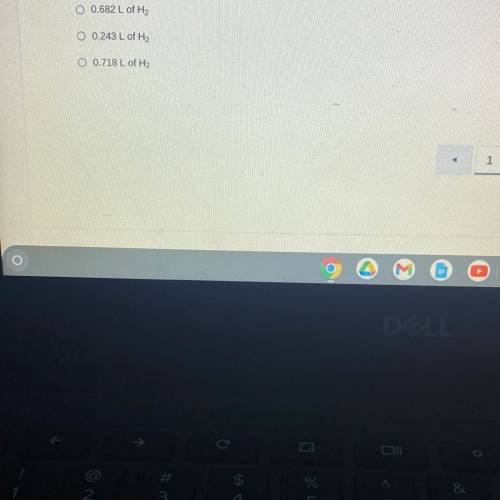
When sodium is placed in water, the following chemical reaction occurs. 2Na(s) + 2H2O (l) —> 2NaOH (aq) + H2 (g). If a teacher uses a 0.500 gram sample of sodium metal for a demonstration with excess water, how many liters of hydrogen gas would be produced at STP?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:00, cynthiagutierrez65
Where can i find naap lab answers sheet/key?
Answers: 1


Chemistry, 22.06.2019 17:00, brandiwingard
What is the mass of phosphorous in a 51-kg person
Answers: 1

Chemistry, 22.06.2019 21:30, starl0rd211
Describe at least two advantages and two disadvantages of using hydropower as a source of energy.
Answers: 2
You know the right answer?
When sodium is placed in water, the following chemical reaction occurs. 2Na(s) + 2H2O (l) —> 2NaO...
Questions in other subjects:


History, 11.05.2021 01:00



English, 11.05.2021 01:00


Mathematics, 11.05.2021 01:00

Mathematics, 11.05.2021 01:00




