
Chemistry, 07.05.2021 22:30 aallyssabrown0120
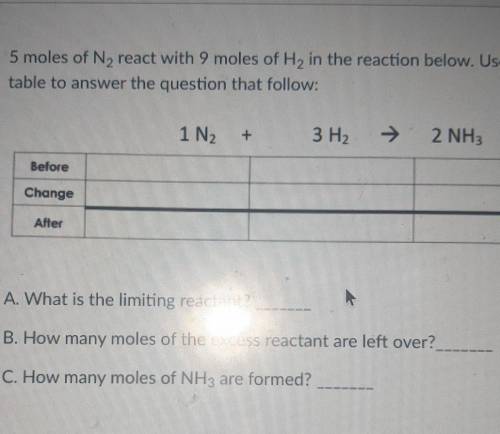
5 moles of N2 react with 9 moles of H2 in the reaction below. Use the BCA table to answer the question that follow: 1N2+3H2=2NH3
A. What is the limiting reactant?
B. How many moles of the excess reactant are left over?
C. How many moles of NH3 are formed?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 21:30, emmalucilleblaha1995
Achemical reaction is done in the setup shown, resulting in a change of mass. what will happen if the same reaction is done in a sealed container that is placed on the electronic balance?
Answers: 1

Chemistry, 22.06.2019 23:00, DESI111609
What is the average rate of the reaction between 10 and 20 s?
Answers: 1

Chemistry, 23.06.2019 04:31, CassidgTab
Which molecules are more strongly attracted to one another -c3h8o molecules that make up liquid rubbing alcohol or ch4 molecules that make up methane gas
Answers: 3
You know the right answer?
5 moles of N2 react with 9 moles of H2 in the reaction below. Use the BCA table to answer the questi...
Questions in other subjects:


Spanish, 09.03.2021 21:40

Mathematics, 09.03.2021 21:40




Mathematics, 09.03.2021 21:40

Biology, 09.03.2021 21:40

History, 09.03.2021 21:40



