
Chemistry, 07.05.2021 22:20 myamiller558
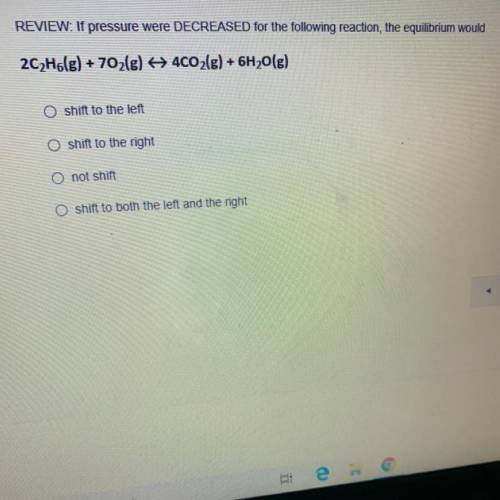
REVIEW: If pressure were DECREASED for the following reaction, the equilibrium would 2C2H6(g) + 702(g) + 4CO2(g) + 6H2O(g)

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:00, youngchapo813p8d9u1
Main expenses you plan on making payments on a new car too. you want to spend 15% of your monthly net pay on the car payment, insurance, registration, and taxes combined. what is your monthly car allowance? $149.46 $298.91 $448.37 $597.83
Answers: 3

Chemistry, 22.06.2019 21:00, taylorlanehart
Use the measurements in the table to determine which unidentified metal has the highest density. metal volume mass a 10.5 cm3 122 g b 14.2 cm3 132 g c 16.1 cm3 115 g d 12.7 cm3 126 g
Answers: 2

Chemistry, 23.06.2019 00:30, kylee65
What are the advantages of using the metric system? designed as a decimal system making conversions simpler more accurate system of measurement has prefixes that correspond to an amount to use with all base units used by the entire scientific community
Answers: 2

You know the right answer?
REVIEW: If pressure were DECREASED for the following reaction, the equilibrium would
2C2H6(g) + 70...
Questions in other subjects:

Mathematics, 05.11.2019 05:31


Mathematics, 05.11.2019 05:31

Mathematics, 05.11.2019 05:31



Physics, 05.11.2019 05:31





