Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 13:00, black99girl
What type of reaction is represented by the following example? 2co2 (g) + 4h2o (l) + 1452 kj 2ch3oh (l) (g) + 3o2 (g) exothermic endothermic
Answers: 1


Chemistry, 22.06.2019 10:00, JOEFRESH10
Suppose the universe were completely empty except for one object-a solid sphere moving through space of 100 km/s. what sort of path would the object be moving in? explain your answer
Answers: 1
You know the right answer?
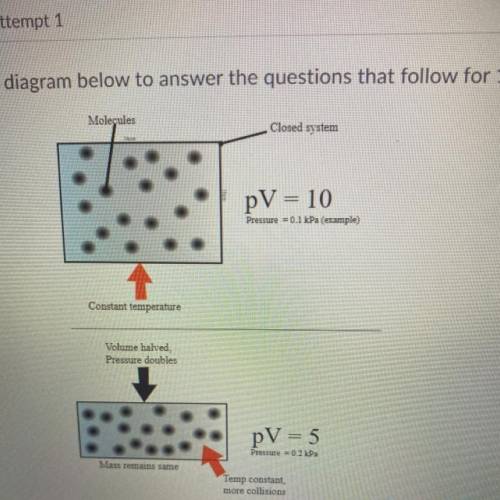
Use the diagram below to answer the question that follow for 1 point each
1. identify what conditi...
Questions in other subjects:


Mathematics, 04.11.2021 02:00

Physics, 04.11.2021 02:00

English, 04.11.2021 02:00




Biology, 04.11.2021 02:00