
Help Quick! 2 questions!
1 The temperature of the areas surrounding Kochi before each storm was about 10°C and there was the same amount of water vapor in the air.
Kochi Before Storm 1: Graphic of a city skyline with an air parcel. The temperature inside the air parcel is 35° Celsius and outside is 10° Celsius. Arrows labeled wind frame the air parcel, pointing inwards. Before Storm 2: Same skyline with an air parcel, framed by the same arrows. The temperature inside the air parcel is 12° Celsius and outside is 10° Celsius. Before Storm 3: Same skyline with an air parcel, but no arrows indicating wind. The temperature inside the air parcel is 35° Celsius and outside is 10° Celsius. Before Storm 4: Same skyline and air parcel, but no arrows indicating wind. The temperature inside the air parcel is 12° Celsius and outside is 10° Celsius.
Given this information, which storm do you predict will have the most rainfall and why?
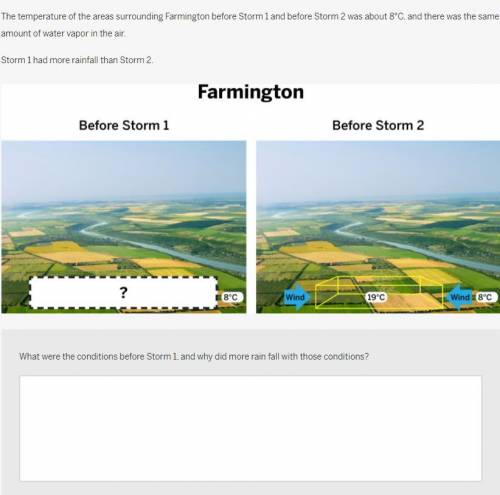
2 The temperature of the areas surrounding Farmington before Storm 1 and before Storm 2 was about 8°C, and there was the same amount of water vapor in the air.
Storm 1 had more rainfall than Storm 2.
Farmington Before Storm 1: Photograph of farmland on a clear day, with a blank response box containing a question mark. The air temperature is 8° Celsius. Before Storm 2: The same scene with an air parcel in place of the blank response box. The temperature inside the air parcel is 19° Celsius and outside is 8° Celsius. Arrows labeled wind frame the air parcel, pointing inwards.
What were the conditions before Storm 1, and why did more rain fall with those conditions?
40points in return


Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 17:10, mikeeway33
Some liquids can be distilled, but only at temperatures that are so high that it is impractical, or so high the compound decomposes. explain why distillation such compounds at significantly less than atmospheric pressure (some degree of vacuum) would solve this problem.
Answers: 2


Chemistry, 22.06.2019 20:20, catchonyet
Which formula equation represents the burning of sulfur to produce sulfur dioxide? s(s) + o2(g) 4502(9) 2h2s(s) + 302(g) —> 2h20(0) + 2502(9) 4fes2+1102 —> 2fe2o3 + 8502 2802(g) + o2(9) v205 , 2503(9)
Answers: 1

Chemistry, 22.06.2019 22:00, jespinozagarcia805
In order to complete this lab. you will need to be familiar with some common chemistry terms. complete the chemical change puzzle and list the relevant terms and their meaning below a. rectant b. product c. supernate
Answers: 3
You know the right answer?
Help Quick! 2 questions!
1 The temperature of the areas surrounding Kochi before each storm was ab...
Questions in other subjects:

English, 08.07.2019 09:30





History, 08.07.2019 09:30



Computers and Technology, 08.07.2019 09:30

Mathematics, 08.07.2019 09:30



