
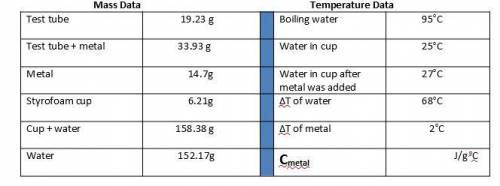
1. Calculate the heat gained by the water (lost by the metal) in the calorimeter using the equation in the introduction.
Metal A
Q = mc(ΔT)
Qwater = -Qmetal
Heat gained = Mass of x Specific heat of x Change in temperature
by the water water (g) water (4.184 J/goC) (ΔT)
The specific heat of the metal can now be calculated:
Specific heat = Heat gained by the water
of metal (c) Mass of metal (g) x ΔT of metal (oC)

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:40, timmonskids6027
Consider the elements bromine and chlorine; which elements has a larger ionic radius ?
Answers: 1

Chemistry, 22.06.2019 06:00, kylieweeks052704
Which of the following did jj thompson discover about atoms? a)an atom has an internal structure. b) atoms are tiny indivisible particles. c)electrons orbit the nucleus of an atom. d) the nucleus of an atom contains protons and neutrons.
Answers: 2

Chemistry, 22.06.2019 06:00, nikejose11
An alkaline battery produces electrical energy according to the following equation. zn(s) + 2 mno2(s) + h2o(l) zn(oh)2(s) + mn2o3(s) (a) determine the limiting reactant if 17.5 g zn and 31.0 g mno2 are used. (type your answer using the format ch4 for ch4.) (b) determine the mass of zn(oh)2 produced. _ g
Answers: 3
You know the right answer?
1. Calculate the heat gained by the water (lost by the metal) in the calorimeter using the equation...
Questions in other subjects:

English, 08.12.2021 17:40


Mathematics, 08.12.2021 17:40


Mathematics, 08.12.2021 17:40





Medicine, 08.12.2021 17:40



