
Chemistry, 05.05.2021 08:40 EllaLovesAnime
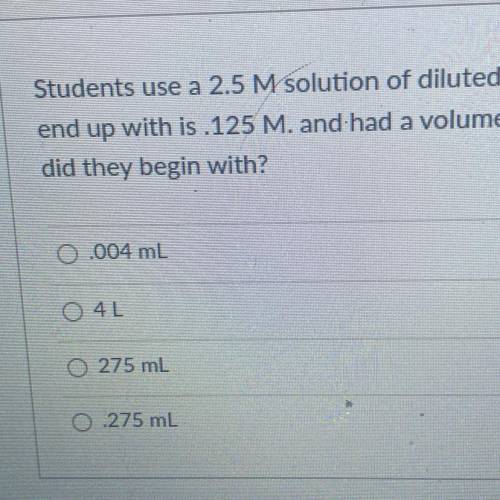
Students use a 2.5 M solution of diluted hydrochloric acid. The Molarity of the solution they
end up with is 125 M. and had a volume of 5500mL. What volume of the concentrated HCI
did they begin with?

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:00, applereams
If a plot weight (in g) vs. volume (in ml) for a metal gave the equation y= 13.41x and r^2=0.9981 what is the density of the metal?
Answers: 2



Chemistry, 23.06.2019 07:40, wrestling2
What is the reduction potential of a hydrogen electrode that is still at standard pressure, but has ph = 5.65 , relative to the she?
Answers: 1
You know the right answer?
Students use a 2.5 M solution of diluted hydrochloric acid. The Molarity of the solution they
end...
Questions in other subjects:

Biology, 05.03.2021 18:10

Mathematics, 05.03.2021 18:10


Mathematics, 05.03.2021 18:10

Mathematics, 05.03.2021 18:10


Mathematics, 05.03.2021 18:10



Mathematics, 05.03.2021 18:10



