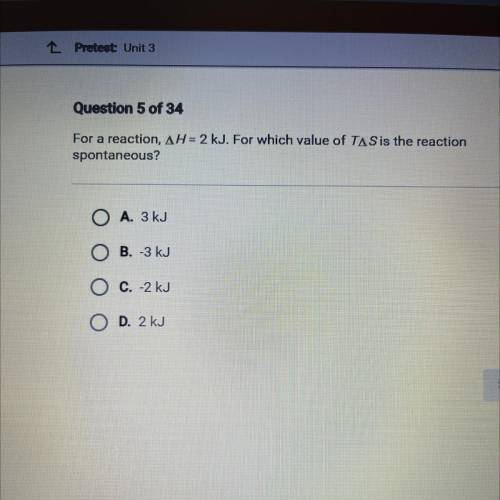
For a reaction, AH = 2 kJ. For which value of TAS is the reaction
spontaneous?
...


Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 17:40, aguilarjose
If 3 moles of a compound use 24 j of energy in a reaction, what is the a hreaction in j/mol?
Answers: 1

Chemistry, 22.06.2019 18:40, johnnysteeler9934
What is one real world example of a colligative property?
Answers: 2


You know the right answer?
Questions in other subjects:

Mathematics, 17.01.2021 17:30


Arts, 17.01.2021 17:30

Mathematics, 17.01.2021 17:30


History, 17.01.2021 17:30




English, 17.01.2021 17:30