
Chemistry, 04.05.2021 23:00 faithlopez209
PLEASE HELP, WILL GIVE BRAINLIEST
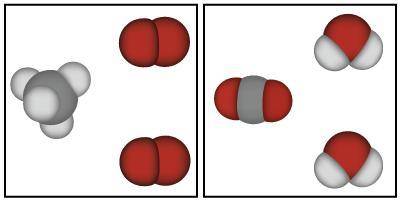
The image below shows models that represent the reactants and products of a chemical reaction.
Based on the image below, which of the following is true?
A. Mass could have either been gained or lost during this chemical reaction because atoms always change mass when they react.
B. Mass was lost in this chemical reaction because the atoms in the reactants are smaller than the atoms in the products.
C. Mass was conserved in this chemical reaction because the same atoms are present in both the products and the reactants.
D. Mass was gained in this chemical reaction because there are more atoms in the products than there were in the reactants.

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 16:50, shaylawaldo11
Which element is least likely to undergo a chemical reaction
Answers: 3

Chemistry, 22.06.2019 21:30, shiannethorn
How many liters of 3.0 m naoh solution will react with 0.60 liters of 4.0 m h2so4? h2so4 + naoh → na2so4 + h2o 1.2 l 1.6 l 2.4 l 2.8 l
Answers: 3


Chemistry, 23.06.2019 08:00, mantha0402
Can anyone answer these questions? ? i need it before 1: 00pm today
Answers: 1
You know the right answer?
PLEASE HELP, WILL GIVE BRAINLIEST
The image below shows models that represent the reactants and pr...
Questions in other subjects:



History, 19.12.2019 01:31







Chemistry, 19.12.2019 01:31



