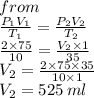Chemistry, 03.05.2021 18:30 katherine3084
3. At 10.0°C and 2.00 atm, the volume of the gas is 75.0 mL. If the temperature of the gas increases to
35.0°C and the pressure decreases to 1.00 atm, what is the new volume?
a. 1630 mL
b. 138 ml
c. 163 mL
d. 0.0017mL

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 14:30, villarrealc1987
In water, a strong acid will break down into its component parts. a. completely b. partly c. never in water, a weak base will break down into its component parts. a. completely b. partly c. never
Answers: 2

Chemistry, 22.06.2019 23:30, johnnysteeler9934
The ammonia molecule in the diagram has the observed bond orientation because
Answers: 1

Chemistry, 23.06.2019 00:00, baseball1525
Which item is most likely part of the safety contract
Answers: 1
You know the right answer?
3. At 10.0°C and 2.00 atm, the volume of the gas is 75.0 mL. If the temperature of the gas increases...
Questions in other subjects:



Mathematics, 24.03.2020 16:12


History, 24.03.2020 16:12

Mathematics, 24.03.2020 16:12

Mathematics, 24.03.2020 16:12

Mathematics, 24.03.2020 16:12


History, 24.03.2020 16:12