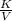What will happen if the pressure of the system is raised to 1.5 atmospheres and the temperature remains same?
The piston will move upwards because the number of moles of the gas particles will increase.
The piston will move downwards because the gas particles will occupy less volume than before.
The piston will move upwards because the gas particles will hit the walls of the container with increased force.
The piston will move downwards because the gas particles will start moving randomly in all directions, pulling the piston down.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 23.06.2019 00:00, PlzNoToxicBan
What is the pressure of 0.500 moles of carbon dioxide gas in a 2.5 l tank and at a temperature of 301 k? (r=0.0821 l·atm/mol·k) 3.08 atm 1.2 atm 0.23 atm 4.01 atm 4.94 atm
Answers: 1

Chemistry, 23.06.2019 00:30, emilylizbeth12334
Which of the following best describes technology a. something created for only scientists to use b. the method of thinking that scientists use. c. the application of engineering to create useful products. c. a scientific idea
Answers: 1

You know the right answer?
What will happen if the pressure of the system is raised to 1.5 atmospheres and the temperature rema...
Questions in other subjects:

Computers and Technology, 01.09.2020 18:01

Biology, 01.09.2020 18:01

Mathematics, 01.09.2020 18:01

English, 01.09.2020 18:01






English, 01.09.2020 18:01