
Chemistry, 30.04.2021 19:40 james22000
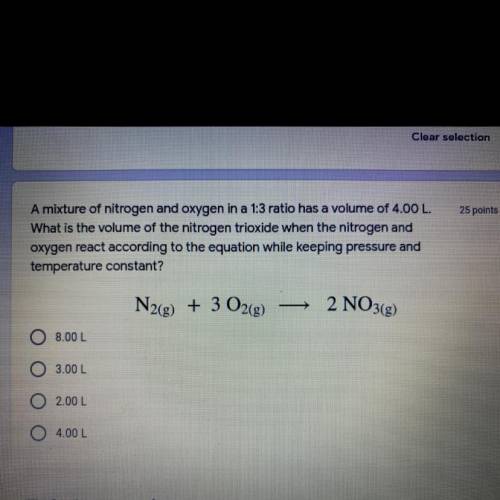
A mixture of nitrogen and oxygen in a 1:3 ratio has a volume of 4.00 L.
What is the volume of the nitrogen trioxide when the nitrogen and
oxygen react according to the equation while keeping pressure and temperature constant?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:10, mpchop
According to the diagram; a) identify the anode of the cell and write the half-reaction that occurs there b) write the overall equation for the reaction that occurs as the cell operates c) calculate the value of the standard cell potential ,e cell. d)write the shorthand notation of the cell above e)indicate the flow of the electrons on the diagram
Answers: 3

Chemistry, 22.06.2019 02:30, bionicboy03120440
What is the mass of sodium in 3 moles of sodium chloride
Answers: 1

Chemistry, 22.06.2019 09:00, kcarstensen59070
What type of energy do chemical bonds have? what type of energy is it converted to during chemical reactions? question 15 options: chemical bonds have kinetic energy, which is converted to potential energy during chemical reactions. chemical bonds have electric energy, which is converted to potential energy during chemical reactions. chemical bonds have heat energy, which is converted to kinetic energy during chemical reactions. chemical bonds have potential energy, which is converted to heat energy during chemical reactions.
Answers: 1

Chemistry, 22.06.2019 18:00, liddopiink1
An object displaces 652 ml of water. the volume of the object is: 0.652 cm³ 6.52 cm³ 65.2 cm³ 652 cm³
Answers: 3
You know the right answer?
A mixture of nitrogen and oxygen in a 1:3 ratio has a volume of 4.00 L.
What is the volume of the...
Questions in other subjects:

Mathematics, 27.01.2022 21:40

Mathematics, 27.01.2022 21:40

History, 27.01.2022 21:40


Mathematics, 27.01.2022 21:40

Mathematics, 27.01.2022 21:40

History, 27.01.2022 21:40

Chemistry, 27.01.2022 21:40


Mathematics, 27.01.2022 21:40



