
Chemistry, 30.04.2021 17:30 janinecastillo01
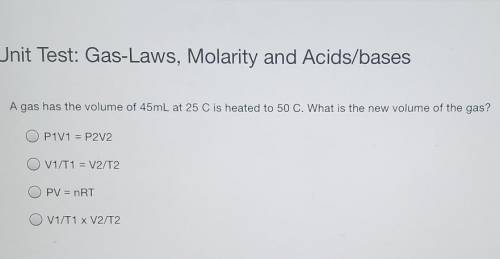
A gas has the volume of 45mL at 25 C is heated to 50 C. What is the new volume of the gas? P1V1 = P2V2 V1/T1 = V2/T2 PV = nRT V1/T1 x V2/T2

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:00, cutebab4786
Consider the point on the plot where 10.0 g of naoh have been added. what amount of naoh, in moles, has been added? 0.308 mol fecl3 initially present
Answers: 1


Chemistry, 22.06.2019 12:30, Svetakotok
Suppose you wanted to make 100 grams of water. what is the molar mass of water (h2o)?
Answers: 2

Chemistry, 22.06.2019 18:00, liddopiink1
An object displaces 652 ml of water. the volume of the object is: 0.652 cm³ 6.52 cm³ 65.2 cm³ 652 cm³
Answers: 3
You know the right answer?
A gas has the volume of 45mL at 25 C is heated to 50 C. What is the new volume of the gas? P1V1 = P2...
Questions in other subjects:

English, 21.10.2020 21:01

Mathematics, 21.10.2020 21:01

English, 21.10.2020 21:01

English, 21.10.2020 21:01


English, 21.10.2020 21:01


Mathematics, 21.10.2020 21:01


Medicine, 21.10.2020 21:01



