
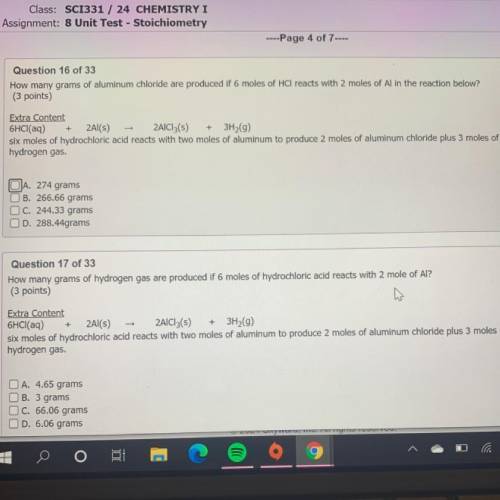
How many grams of aluminum chloride are produced if 6 moles of HCl reacts with 2 moles of Al in the reaction below?
(3 points)
Extra Content
6HCl(aq) + 2Al(s) - 2AlCl3(s) + 3H2(9)
six moles of hydrochloric acid reacts with two moles of aluminum to produce 2 moles of aluminum chloride plus 3 moles of
hydrogen gas.
JA. 274 grams
B. 266.66 grams
C. 244.33 grams
D. 288.44grams

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:00, stephstewart1209
Which of the following observations indicates that there is a small, dense, positively charged part in the center of an atom? some uncharged particles are scattered by a gold foil. all uncharged particles are attracted towards a gold foil. all positively charged particles pass straight through a gold foil. some positively charged particles bounce back from a gold foil.
Answers: 2



Chemistry, 23.06.2019 07:00, ashleyrturner08
Explain what happened when the storm surges from hurricanes reached the gulf coast
Answers: 1
You know the right answer?
How many grams of aluminum chloride are produced if 6 moles of HCl reacts with 2 moles of Al in the...
Questions in other subjects:





Mathematics, 12.12.2020 21:00


Business, 12.12.2020 21:00

Mathematics, 12.12.2020 21:00

Health, 12.12.2020 21:00

Social Studies, 12.12.2020 21:00



