Question:
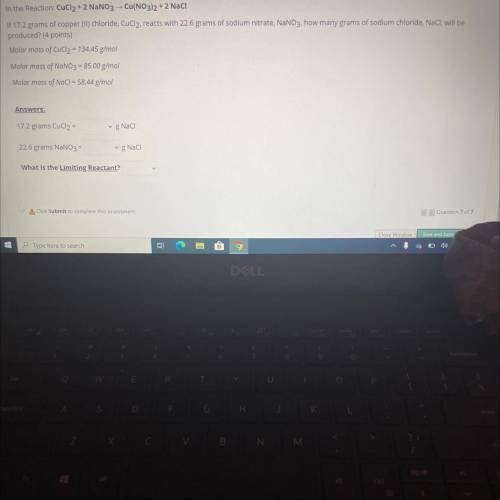
In the Reaction: CuCl2 + 2 NaNO3 -- Cu(NO3)2 + 2 Naci
If 17.2 grams of copper (II)...

Chemistry, 29.04.2021 19:40 quickestlearner8562
Question:
In the Reaction: CuCl2 + 2 NaNO3 -- Cu(NO3)2 + 2 Naci
If 17.2 grams of copper (II) chloride, CuCl2, reacts with 22.6 grams of sodium nitrate, NaNO3. how many grams of sodium chloride, NaCl, will be
produced? (4 points)
Molar mass of CuCl2 = 134.45 g/mol
Molar mass of NaNO3 = 85.00 g/mol
Molar mass of NaCl = 58.44 g/mol
Answers:
17.2 grams CuCl2 =
gNaci
v
22.6 grams NaNO3 =
g Naci
What is the Limiting Reactant?
No

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:00, cathydaves
What is the chemical formula of the following compound
Answers: 1

Chemistry, 21.06.2019 19:30, haybaby312oxdjli
Water molecules have a strong attraction to each other because of hydrogen bonding, allowing water to move against gravity up a plant's stem through capillary action. true false
Answers: 2

Chemistry, 22.06.2019 12:30, Svetakotok
Suppose you wanted to make 100 grams of water. what is the molar mass of water (h2o)?
Answers: 2

Chemistry, 22.06.2019 17:20, alexis3060
How do you know when a chemical reaction has occurred
Answers: 1
You know the right answer?
Questions in other subjects:

Biology, 18.08.2019 11:30


Chemistry, 18.08.2019 11:30


Mathematics, 18.08.2019 11:30

Mathematics, 18.08.2019 11:30

Chemistry, 18.08.2019 11:30





