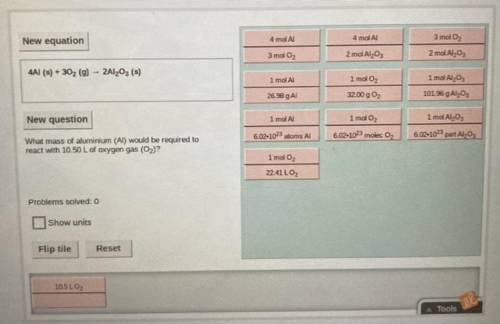
Equation: 4Al (s) + 3O2 (g) —> 2Al2O3 (s)
(Solve using stoichiometry)
...

Chemistry, 29.04.2021 01:00 student0724
Equation: 4Al (s) + 3O2 (g) —> 2Al2O3 (s)
(Solve using stoichiometry)

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 14:50, alexabbarker9781
How are evaporation and sublimation similar? a both involve the formation of a gas. b both release energy to the surroundings. c both take place throughout a solid. d both take place at the surface of a liquid.
Answers: 1

Chemistry, 22.06.2019 18:00, rodriguezscarlet1713
Hydrogenation reactions, in which h2 and an "unsaturated" organic compound combine, are used in the food, fuel, and polymer industries. in the simplest case, ethene (c2h4) and h2 form ethane (c2h6). if 140 kj is given off per mole of c2h4 reacting, how much heat (in mj) is released when 12 kg of c2h6 forms?
Answers: 2

Chemistry, 22.06.2019 19:50, ellycleland16
Identify the lewis base in this balanced equation: fe3+ h2o fe(h2o)63+
Answers: 1
You know the right answer?
Questions in other subjects:




Mathematics, 24.01.2022 23:20

English, 24.01.2022 23:20

Mathematics, 24.01.2022 23:20

Mathematics, 24.01.2022 23:20

Mathematics, 24.01.2022 23:20

Mathematics, 24.01.2022 23:20

History, 24.01.2022 23:20



