Answers: 1


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 13:30, nasibamurodova
What are the chemical names of these compounds? ke: mg3n2: reset next
Answers: 1

Chemistry, 22.06.2019 20:30, camerondillonn
Calculate the percent composition by mass of each element in al(oh)3. use at least three significant figures.
Answers: 1
You know the right answer?
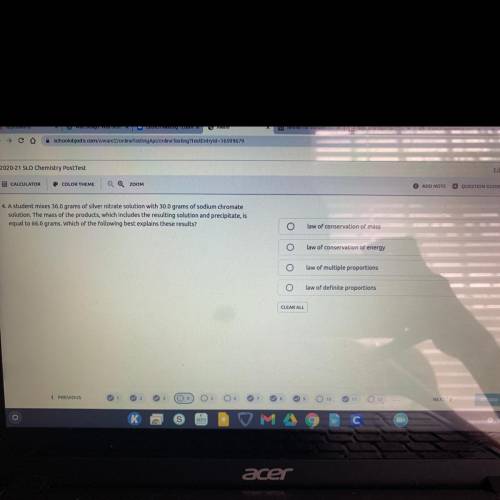
A student mixes 36.0 grams of silver nitrate solution with 30.0 grams of sodium chromate solution. T...
Questions in other subjects:


Mathematics, 22.04.2021 04:10

English, 22.04.2021 04:10

Mathematics, 22.04.2021 04:10


Mathematics, 22.04.2021 04:10


Mathematics, 22.04.2021 04:10

Mathematics, 22.04.2021 04:10

History, 22.04.2021 04:10