NEED ANDWER ASAP
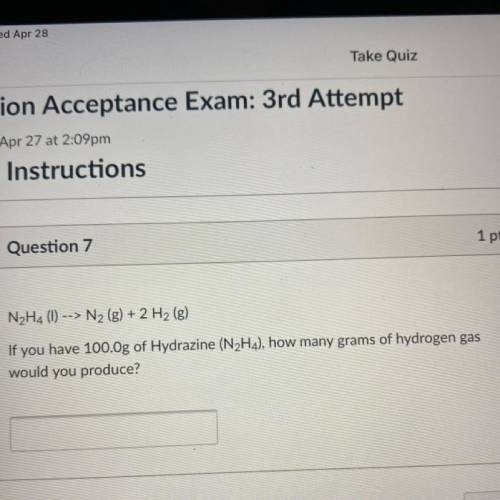
N2H4 (1) --> N2 (g) + 2 H2 (g)
If you have 100.Og of Hydrazine (N2H4), h...

Chemistry, 28.04.2021 20:10 kaywendel2008
NEED ANDWER ASAP
N2H4 (1) --> N2 (g) + 2 H2 (g)
If you have 100.Og of Hydrazine (N2H4), how many grams of hydrogen gas
would you produce?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:00, mercymain1014
An excess of lithium oxide undergoes a synthesis reaction with water to produce lithium hydroxide li2o+h2o→2lioh if 1.05 g of water reacted, what is the theoretical yield of lithium hydroxide? a) 5.83 x 10–2 g lioh b) 1.17 x 10–1 g lioh c) 2.79 x 100 g lioh d) 1.40 x 100 g lioh
Answers: 1

Chemistry, 22.06.2019 14:30, CoolRahim9090
Which of the following represents the ester functional group? a. -coo- b. -cho c. -cooh d. c=o
Answers: 1

Chemistry, 23.06.2019 13:30, jharrington583
Which of the following is true regarding chemical and nuclear reactions?
Answers: 1
You know the right answer?
Questions in other subjects:

Mathematics, 16.07.2019 10:30


Mathematics, 16.07.2019 10:30




Mathematics, 16.07.2019 10:30



Mathematics, 16.07.2019 10:30



