Please
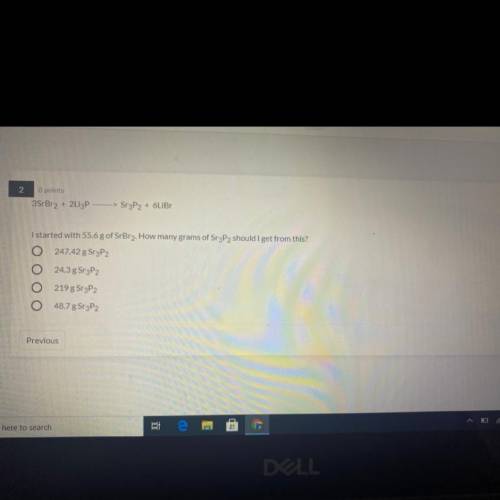
3SrBr2 + 2Li3P —>Sr3P2 + 6LiBr
I started with 55.6 g of SrBr2. How many gram...

Chemistry, 28.04.2021 19:30 rwlockwood1
Please
3SrBr2 + 2Li3P —>Sr3P2 + 6LiBr
I started with 55.6 g of SrBr2. How many grams of Sr3P2 should I get from this?
A) 247.42 g SrP2
B) 24.3 g Sr3P2
C) 219 g Sr3P2
D) 48.7 g Sr3P2

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 23.06.2019 02:50, igraha17
Dumbledore decides to gives a surprise demonstration. he starts with a hydrate of na2co3 which has a mass of 4.31 g before heating. after he heats it he finds the mass of the anhydrous compound is found to be 3.22 g. he asks everyone in class to determine the integer x in the hydrate: na2co3·xh2o; you should do this also. round your answer to the nearest integ
Answers: 2

Chemistry, 23.06.2019 06:30, SimplyGenesis762
Generally, observed behavior that can be formulated into a statement, sometimes mathematical in nature, is called a(n): a. observation. b. measurement. c. theory. d. natural law. e. experiment.
Answers: 2

Chemistry, 23.06.2019 12:30, ella3714
17) large amounts of very important metal titanium are made by reacting magnesium metal with titanium tetrachloride. titanium metal and magnesium chloride are produced. a) write the balanced equation for this reaction. b) how many kilograms of magnesium are required to produce 1.00 kilograms of titanium? ( show work, .)
Answers: 1
You know the right answer?
Questions in other subjects:




Geography, 20.07.2019 06:00

English, 20.07.2019 06:00




Mathematics, 20.07.2019 06:00

History, 20.07.2019 06:00



