Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:50, AysiaRamosLee
What is the temperature of one mole of helium gas at stp?
Answers: 3

Chemistry, 22.06.2019 04:30, coryoddoc3685
Turbo the snail moves across the ground at a pace of 12 feet per day. if the garden is 48 feet away, how many days will it take for the snail to get there?
Answers: 2

Chemistry, 23.06.2019 06:30, lainnn974
(04.01 lc) which of the following is true about science? (5 points) select one: a. it is not influenced by social conditions. b. it is not determined by external local factors. c. political conditions are unable to influence it. d. economic concerns may prevent it from solving problems.
Answers: 1

Chemistry, 23.06.2019 08:10, 20dyeaubn
Time remaining 58: 10 an atom that has 84 protons and 86 neutrons undergoes a reaction. at the end of the reaction, it has 82 protons and 84 neutrons. what happened to the atom? it accepted radiation in a chemical reaction it donated neutrons to another atom in a chemical reaction it emitted an alpha particle in a nuclear reaction. it accepted protons in a nuclear reaction. mark this and retum save and exit next submit
Answers: 3
You know the right answer?
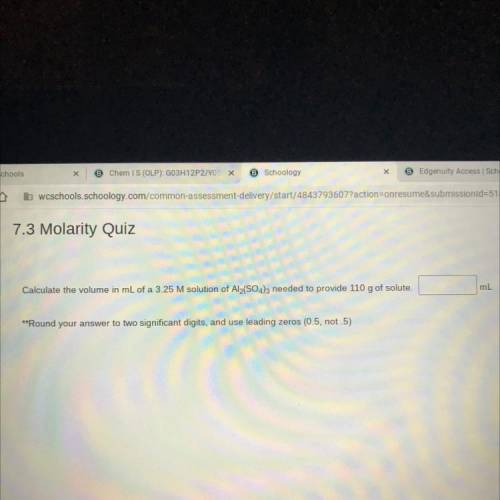
Calculate the volume in mL of a 3.25 M solution of Al2(SO4)3 needed to provide 110 g of solute.
Questions in other subjects:


Mathematics, 27.06.2019 15:30


Mathematics, 27.06.2019 15:30



History, 27.06.2019 15:30


Mathematics, 27.06.2019 15:30

Mathematics, 27.06.2019 15:30