
Chemistry, 28.04.2021 03:00 donaji1024perez
Determine the equilibrium constant for the above reaction if the concentration for N20 = 150 X 10-2M and NO2 = 5.70 X 10-2M.
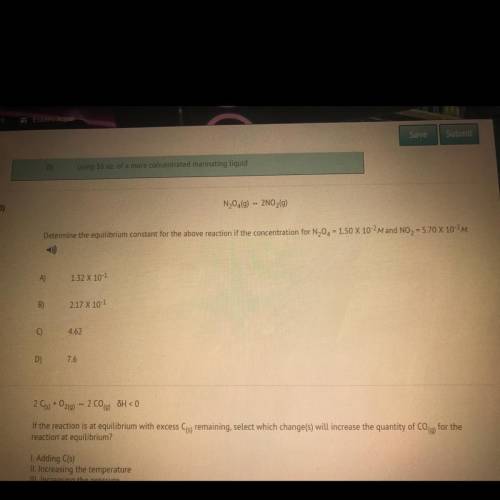

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 14:30, twinkieslayer
As a part of an experiment a student burns propane to produce carbon dioxide and water she remembers that she must follow the law conservation of matter when writing a balanced chemical equation which of these equation adheres to the law of conservation of matter
Answers: 1

Chemistry, 22.06.2019 10:30, kluckey3426
Asample of air with a volume of 2.20m3 at a pressure of 105 kpa and a temperature of 30c is cooled to 10c and the pressure is reduced to 75.0 kpa. what is the new volume? 6.9 1.34 2.56 43.0 2.88
Answers: 1


Chemistry, 22.06.2019 20:00, kalcius9698
What is the molarity of the solution produced when 145 g of nacl is dissolved in sufficient water to prepare 2.75 l of solution?
Answers: 1
You know the right answer?
Determine the equilibrium constant for the above reaction if the concentration for N20 = 150 X 10-2M...
Questions in other subjects:



Social Studies, 31.01.2020 04:43







History, 31.01.2020 04:43



