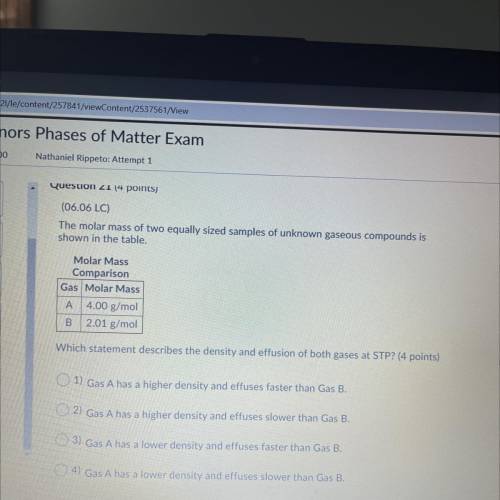
(06.06 LC)
The molar mass of two equally sized samples of unknown gaseous compounds is
shown...


Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 23:00, brianfranklin17
What is the correct lewis dot structure for arsenic?
Answers: 2

Chemistry, 23.06.2019 00:30, mariaramirez110379
On the periodic table, elements are arranged by which of the following. a. mass numbers. b. increasing atomic number. c. alphabetical order. or d. density
Answers: 1
You know the right answer?
Questions in other subjects:

Social Studies, 21.08.2019 04:30

Social Studies, 21.08.2019 04:30




Mathematics, 21.08.2019 04:30

English, 21.08.2019 04:30

History, 21.08.2019 04:30

Mathematics, 21.08.2019 04:30