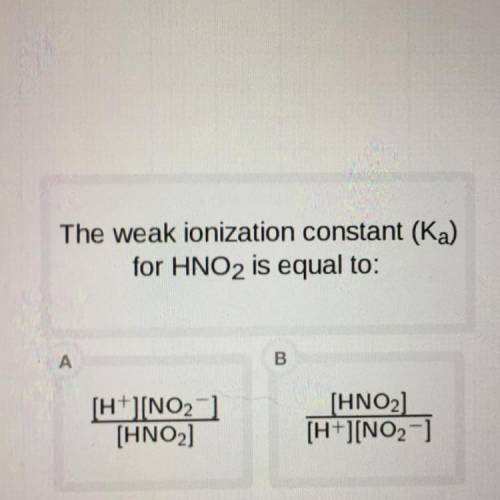
The weak ionization constant (Ka)
for HNO2 is equal to:
...

Chemistry, 27.04.2021 04:10 inucornspineapple
The weak ionization constant (Ka)
for HNO2 is equal to:

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:30, leslyrivera11
If i make a solution by adding 83grams of sodium hydroxide to 750ml i’d water what is the molarity of sodium hydroxide
Answers: 1

Chemistry, 22.06.2019 01:30, arodavoarodavo
The table lists pressure and volume values for a particular gas. which is the best estimate for the value of v at p = 7.0 × 103 pascals?
Answers: 3

You know the right answer?
Questions in other subjects:


History, 26.10.2020 16:20

Spanish, 26.10.2020 16:20

English, 26.10.2020 16:20

Mathematics, 26.10.2020 16:20

Health, 26.10.2020 16:20

English, 26.10.2020 16:20


Spanish, 26.10.2020 16:20

History, 26.10.2020 16:20



