
Chemistry, 27.04.2021 02:10 coolgirl5679
Please help
The thermochemical equation for the combustion of propane gas is:
CH4 (g) + 2O2 (g) CO2 (g) + 2H2O (I), ΔH = -890 kJ/mol
Calculate much heat is released when 3.5 moles of propane have a combustion reaction.
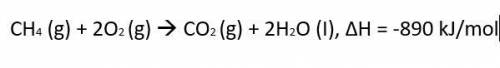

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 18:00, kingamir
Answer asap need it by wednesday morning carry out the following calculations on ph and ka of from data. i. calculate the ph of 0.02m hcl ii. calculate the ph of 0.036m naoh iii. calculate the ph of 0.36m ca(oh)2 iv. calculate the ph of 0.16m ch3cooh which has ka = 1.74 x 10-5 mol dm-3 v. calculate ka for weak acid ha which has a ph of 3.65 at 0.30m concentration vi. calculate the ka of a solution made by mixing 15.0 cm3 0.2m ha and 60.0 cm3 0.31m a-. [ph= 3.80] vii. calculate the ph of a solution made by mixing 15.0 cm3 0.1m naoh and 35.0 cm3 0.2m hcooh. [ka = 1.82 x 10-4 m]
Answers: 1

Chemistry, 22.06.2019 21:50, isabel81ie
Given the data below for the reaction, 2 a + 2 b + 4 c => d + e + 3 f, the reaction is fill in the [ ] order in a, fill in the [ ] order in b, fill in the [ ] order in c and fill in the [ ] order overall. (use the words "first, second, third, fourth" to fill each blank)experimentinitial conc of a, mol/l initial conc of b, mol/l initial conc of c, mol/l initial rate, mol/l. s1 0.1 0.1 0.2 2 x 10-32 0.2 0.3 0.2 6 x 10-33 0.3 0.1 0.2 2 x 10-34 0.4 0.3 0.4 1.2 x 10-2
Answers: 2
You know the right answer?
Please help
The thermochemical equation for the combustion of propane gas is:
CH4 (g)...
CH4 (g)...
Questions in other subjects:

Mathematics, 16.10.2020 22:01

History, 16.10.2020 22:01

Mathematics, 16.10.2020 22:01

Mathematics, 16.10.2020 22:01

German, 16.10.2020 22:01



Mathematics, 16.10.2020 22:01





