
Chemistry, 26.04.2021 22:10 DESI111609
Calculating partial pressure in a gas mixture
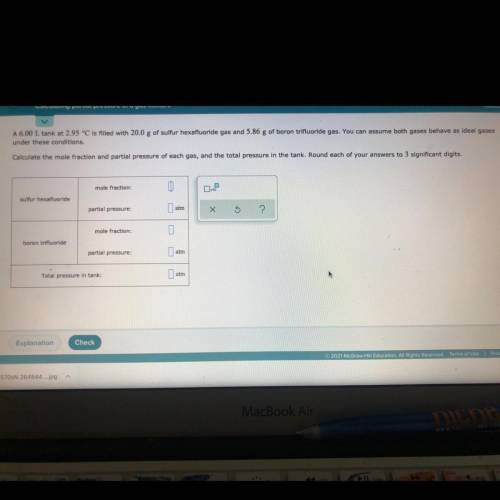
A 6.00 L tank at 2.95 °C is filled with 20.0 g of sulfur hexafluoride gas and 5.86 g of boron trifluoride gas. You can assume both gases behave as ideal gases
under these conditions.
Calculate the mole fraction and partial pressure of each gas, and the total pressure in the tank. Round each of your answers to 3 significant digits.
mole fraction:
DI
sulfur hexafluoride
partial pressure:
atm
Х
5
?
mole fraction:
0
boron trifluoride
partial pressure:
atm
Total pressure in tank:

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:50, mayamabjishovrvq9
Why doesn't heat added to water make the tempature rise above 100c
Answers: 2


You know the right answer?
Calculating partial pressure in a gas mixture
A 6.00 L tank at 2.95 °C is filled with 20.0 g of su...
Questions in other subjects:


History, 29.10.2020 20:40

Biology, 29.10.2020 20:40


Biology, 29.10.2020 20:40


History, 29.10.2020 20:40



History, 29.10.2020 20:40



