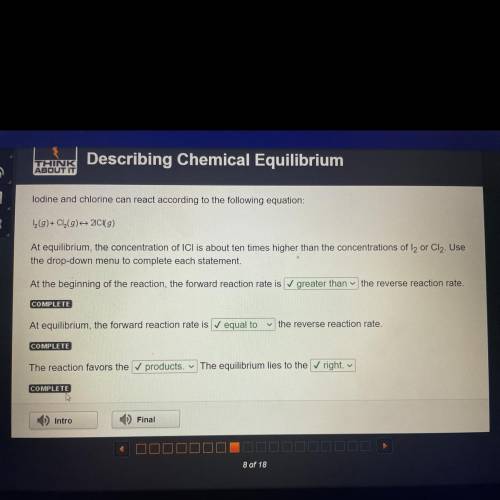
Lodine and chlorine can react according to the following equation:
1 (9)+ Cl(9) 20 (9)
At eq...

Chemistry, 26.04.2021 07:40 kinglightskin2k
Lodine and chlorine can react according to the following equation:
1 (9)+ Cl(9) 20 (9)
At equilibrium, the concentration of ICI is about ten times higher than the concentrations of l2 or Cl2. Use
the drop-down menu to complete each statement.
At the beginning of the reaction, the forward reaction rate is greater than the reverse reaction rate.
COMPLETE
At equilibrium, the forward reaction rate is equal to
the reverse reaction rate.
COMPLETE
The reaction favors the ✓ products. The equilibrium lies to the right.
COMPLETE

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:50, justabeachbum
If a reactant was removed, did the new equilibrium system shift to make more reactants or more products?
Answers: 1

Chemistry, 22.06.2019 16:30, danbelucio
Explain in detail of the four major scientific developments that spurred the formulation of the plate tectonics theory
Answers: 2

Chemistry, 23.06.2019 02:30, puppylover72
Calculate the ph at the equivalence point for the titration of a solution containing 150.0 mg of ethylamine (c2h5nh2) with 0.1000 m hcl solution. the volume of the solution at the equivalence point is 250.0 ml. kb forethylamine is 4.7 × 10−4 .
Answers: 2

Chemistry, 23.06.2019 11:20, zackarygonzalez1028
Try to reduce the amount of leftover ingredients by changing the amount of one, two, or all three starting ingredients. show your stoichiometric calculations below. water 946.36 g sugar 196.86 g lemon juice193.37 g
Answers: 2
You know the right answer?
Questions in other subjects:


Mathematics, 11.01.2020 04:31

History, 11.01.2020 04:31

Mathematics, 11.01.2020 04:31

Mathematics, 11.01.2020 04:31

Chemistry, 11.01.2020 04:31


Computers and Technology, 11.01.2020 04:31




