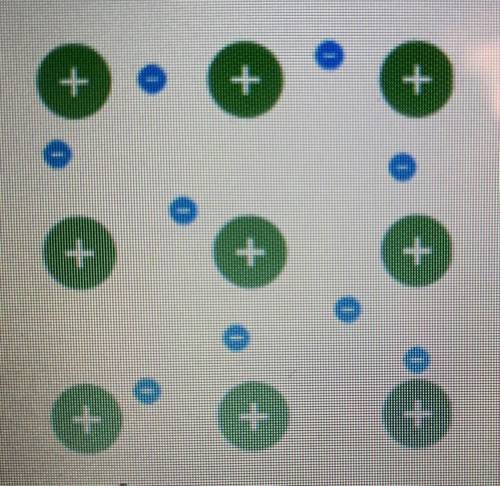
Examine the model of a metallic bond.
What do the smaller circles represent?
o the cations h...

Examine the model of a metallic bond.
What do the smaller circles represent?
o the cations held in place by the attractive forces within the sea of electrons
O the delocalized electrons that are free to move around the positive cations
the distinct and rigid bond between the different metal atoms
the unshared pairs of electrons of the metal atoms involved in the metallic bond

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:00, officialgraciela67
What is the volume occupied by 10.0 dm3 of gas at standard pressure after it has been compressedat constant temputure to 500.0 kpa?
Answers: 1

Chemistry, 22.06.2019 08:40, kellymcdow5135
For each of the following compounds, write the formula then predict whether it would be a strong, weak, or non-electrolyte when placed in di water. for the ionic compounds only, put (s) or (aq) after the forrmula formula strong, weak or non electrolyte? a calcium hydroxide b. silver carbonate c. lead(ii) sulfate d. phosphorus trifluoride e. sodium phosphide f barium sulfate g. strontium acetate h. zinc nitrate
Answers: 3


Chemistry, 22.06.2019 11:50, hamidaakter936848
Calculate the molarity of each of the following solutions. part a) 0.12 mol of lino3 in 5.5 l of solution part b) 60.7 g c2h6o in 2.48 l of solution part c) 14.2 mg ki in 100 ml of solution
Answers: 2
You know the right answer?
Questions in other subjects:

Geography, 20.05.2021 22:10

History, 20.05.2021 22:10

Business, 20.05.2021 22:10


Mathematics, 20.05.2021 22:10

Mathematics, 20.05.2021 22:10

English, 20.05.2021 22:10

English, 20.05.2021 22:10

History, 20.05.2021 22:10

Mathematics, 20.05.2021 22:10



