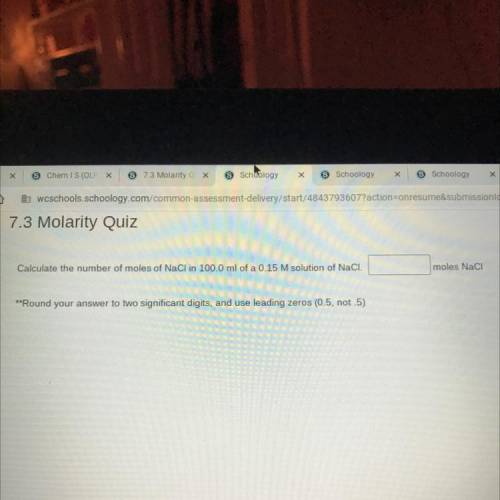
Calculate the number of moles of NaCl in 100.0 ml of a 0.15 M solution of NaCl.
...

Chemistry, 24.04.2021 02:00 yaaaaa31gghgf
Calculate the number of moles of NaCl in 100.0 ml of a 0.15 M solution of NaCl.

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 09:20, UsedForSchool2018
Which of these statements explains the difference between nuclear binding energy and the strong nuclear force ?
Answers: 3

Chemistry, 22.06.2019 23:50, josie311251
Be sure to answer all parts. the following equilibrium constants were determined at 1123 k: c(s) + co2(g) ⇌ 2co(g) k'p = 1.30 × 1014 co(g) + cl2(g) ⇌ cocl2(g) k''p = 6.00 × 10−3 calculate the equilibrium constant at 1123 k for the reaction: c(s) + co2(g) + 2cl2(g) ⇌ 2cocl2(g) 4.68 × 10 9 (enter your answer in scientific notation.) write the equilibrium constant expression, kp:
Answers: 3
You know the right answer?
Questions in other subjects:

Mathematics, 05.11.2020 17:00

Mathematics, 05.11.2020 17:00










