
Chemistry, 24.04.2021 01:00 kayla114035
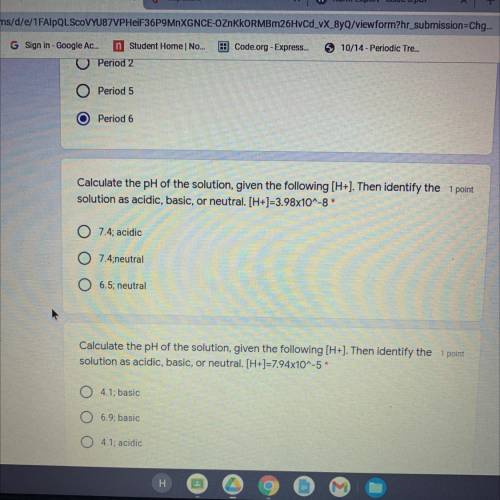
Calculate the pH of the solution, given the following (H+). Then identify the 1 point
solution as acidic, basic, or neutral. [H+]=3.98x10^-8*
O 7.4; acidic
7.4;neutral
6.5; neutral
Calculate the pH of the solution, given the following (H+). Then identify the 1 point
solution as acidic, basic, or neutral. [H+]=7.94x10^-5*

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, larreanathalie3523
The diagram shows the structures of horse and cat forelimbs. what does the diagram suggest about the evolutionary relationship between these two mammals? a. they have homologous structures, indicating a common ancestor. b. they have analogous structures, indicating a common ancestor. c. they have homologous structures, indicating that they do not have a common ancestor. d. they have analogous structures, indicating that they do not have a common ancestor.
Answers: 2


Chemistry, 22.06.2019 13:30, makenziehook8
Which is true of a liquid? it has a definite volume but not a definite mass. it has a definite mass but not a definite volume. it has a definite volume but not a definite shape. it has a definite shape but not a definite volume.
Answers: 2

Chemistry, 22.06.2019 21:00, Janznznz4012
Once similarity and one difference between a mixture of elements and a mixture of compounds
Answers: 3
You know the right answer?
Calculate the pH of the solution, given the following (H+). Then identify the 1 point
solution as...
Questions in other subjects:

Chemistry, 13.02.2022 17:20

Mathematics, 13.02.2022 17:20

Mathematics, 13.02.2022 17:20

English, 13.02.2022 17:20


Chemistry, 13.02.2022 17:20


Mathematics, 13.02.2022 17:20

Mathematics, 13.02.2022 17:20

Social Studies, 13.02.2022 17:20



