
Chemistry, 23.04.2021 22:40 ibarral37102
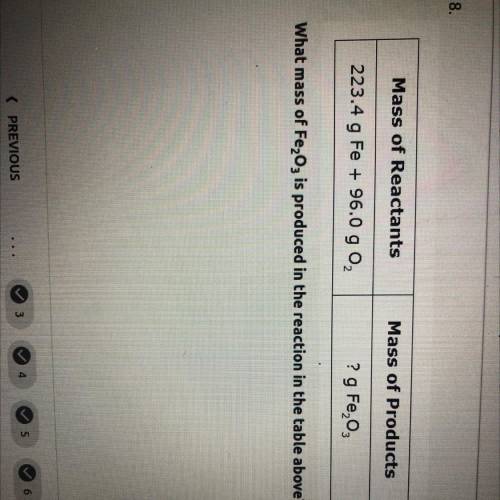
what mass of Fe2O3 is produced in the reaction in the table above mass of reactants= 223.4 g Fe+96.0 g O2. and mass of products=? g Fe2O3

Answers: 2


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 00:00, 2024daisjavien
Which actions would increase the rate at salt dissolves in water? stir the water? crush the salt? use less water? heat the water? cool the salt
Answers: 3


Chemistry, 22.06.2019 05:50, zaleemawhite
Significant figures are digits read directly from the measuring instrument plus one more digit, which is __ by the observer.
Answers: 2
You know the right answer?
what mass of Fe2O3 is produced in the reaction in the table above mass of reactants= 223.4 g Fe+96.0...
Questions in other subjects:



World Languages, 09.04.2020 00:17

Arts, 09.04.2020 00:17



Mathematics, 09.04.2020 00:17





