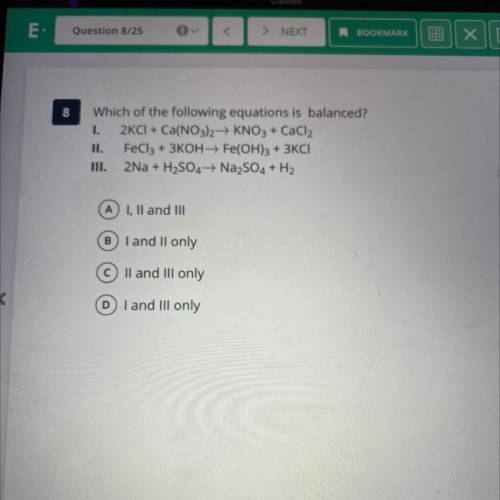
Which of the following equations is balanced?
I. 2KCI + Ca(NO3)2 → KNO3 + CaCl2
II. FeCl3 +...

Chemistry, 23.04.2021 20:10 chryssiem16
Which of the following equations is balanced?
I. 2KCI + Ca(NO3)2 → KNO3 + CaCl2
II. FeCl3 + 3KOH+ Fe(OH)3 + 3KCI
TII. 2Na + H2SO4 → Na2SO4 + H2
A) I, II and III
B I and II only
C II and III only
D I and III only

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:00, pettygirl13
Describe the particles of all three phases of matter in the root beer float. (how are the particles arranged and moving? )
Answers: 3

Chemistry, 21.06.2019 23:30, 23gordns
Problem #3 (ch. 1, problem 15)the ideal gas law provides one way to estimate the pressure exerted by a gas on a container. the law isí‘ťí‘ť=푛푛푛푛푛푛푉푉mo re accurate estimates can be made with the van der waals equationí‘ťí‘ť=í‘›í‘›í‘›í‘›í‘›í‘›í‘ ‰í‘‰â’푛푛푟푟â’푞푞푛푛2í‘ ‰í‘‰2where the term nb is a correction for the volume of the molecules and the term an2/v2is a correction for molecular attractions. the values of a and b depend on the type of gas. the gas constant is r, the absolutetemperature is t, the gas volume is v, and the number of moles of gas molecules is indicated by n. if n = 1 mol of an ideal gas were confined to a volume of v = 22.41 l at a temperature of 0â°c (273.2k), it would exert a pressure of 1 atm. in these units, r = 0.0826.for chlorine gas (cl2), a = 6.49 and b = 0.0562. compare the pressure estimates given by the ideal gas law and the van der waals equation for 1 mol of cl2 in 22.41 l at 273.2 k. what is the main cause of the difference in the two pressure estimates, the molecular volume or the molecular attractions?
Answers: 1

Chemistry, 22.06.2019 00:30, portedon8644
13. calculate the initial concentration (before precipitation) of carbonate ions after the addition of each 0.05 ml of solution b to the 1.00 l beaker of solution a. divide the work among group members and write the answers in the table in model 3. assume the volume change as solution b is added is negligible. 14. notice the initial concentrations of zn2+ - and cu2+ in the table in model 3. a. explain how these were obtained from the data in model 2. b. as solution b is added and precipitates form, do these initial concentrations change? 15. use the data in model 2 to indicate the presence of precipitate (either znco3 or cuco3) after each 0.05 ml addition of solution b in model 3. 16. use the initial concentrations of carbonate ions and zinc ions to calculate the reaction quotient, qsp for the zinc carbonate scenarios in model 3. divide the work among group members and write the answers in the table in model 3. 17. use the initial concentrations of carbonate ion and copper(ii) ions to calculate the qsp for the copper(ii) carbonate scenarios in model 3. divide the work among group members and write the answers in the table in model 3.
Answers: 3

Chemistry, 22.06.2019 07:30, deidaraXneji
Using data from seismic waves, geologists have learned that earth’s interior is made up of several
Answers: 3
You know the right answer?
Questions in other subjects:


Chemistry, 06.05.2020 11:59

Mathematics, 06.05.2020 11:59

Mathematics, 06.05.2020 11:59


French, 06.05.2020 11:59

Mathematics, 06.05.2020 11:59


Mathematics, 06.05.2020 11:59

Chemistry, 06.05.2020 11:59



