
Chemistry, 23.04.2021 08:50 kemzzoo7206
PLEASE JUST DONT POST A FILE AS AN ANSWER, ANYWAYS HALP
For parts of the free-response question that require calculations, clearly show the method used and the steps involved in arriving at your answers. You must show your work to receive credit for your answer. Examples and equations may be included in your answers where appropriate.
Answer the following questions related to H2O .
a. Using the information in the table above, determine the value of ΔG° at 298K for the process represented by the equation H2O(l)⇄H2O(g) .
b. Considering your answer to part (a), indicate whether the process is thermodynamically favorable at 298K . Justify your answer.
c. Considering your answer to part (b), explain why H2O(l) has a measurable equilibrium vapor pressure at 298K .
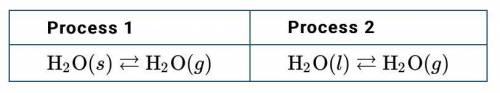
Water vapor can be produced in two different processes, as represented below.
d. In terms of concepts of entropy and the particle-level structure of the different phases of water, explain why the change in entropy, ΔS , is greater for process 1 than for process 2.


Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, connienash95
Explain why scientists use shared characteristics to make cladograms.
Answers: 1

Chemistry, 22.06.2019 10:30, Clivensp5
Skills of homo sapiens were found an excavation. the skulls were preserved because the bodies were frozen. so, these fossils are (blank) fossils. the image shows the evolution of skulls beginning 2 to 3 million years ago. based on the image, modern human skulls(blank) ape skulls.
Answers: 1

Chemistry, 22.06.2019 14:00, JJlover1892
Ascientist measures the speed of sound in a monatomic gas to be 449 m/s at 20∘c. what is the molar mass of this gas?
Answers: 2

Chemistry, 22.06.2019 14:30, CoolRahim9090
Which of the following represents the ester functional group? a. -coo- b. -cho c. -cooh d. c=o
Answers: 1
You know the right answer?
PLEASE JUST DONT POST A FILE AS AN ANSWER, ANYWAYS HALP
For parts of the free-response question th...
Questions in other subjects:




Chemistry, 25.08.2019 00:30


Mathematics, 25.08.2019 00:30

Mathematics, 25.08.2019 00:30

History, 25.08.2019 00:30




