
Chemistry, 22.04.2021 18:20 brennae8529
If the formula of ammonium nitrate is NH4NO3, determine how many moles of the solid were dissolved in the water in the experiment #2.
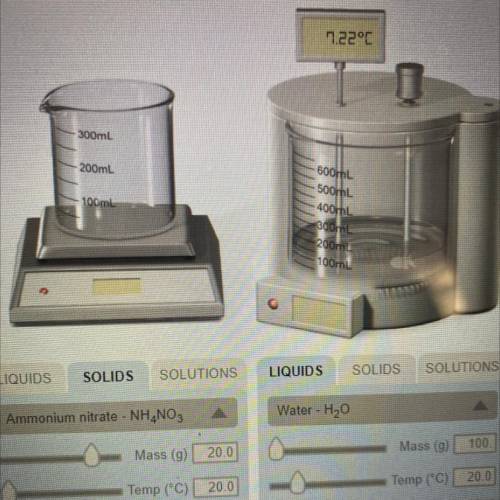

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 16:20, kingsqueen883
Consider the two electron arrangements for neutral atoms a and b. are atoms a and b the same element? a - 1s2, 2s2, 2p6, 3s1 b - 1s2, 2s2, 2p6, 5s1
Answers: 3

Chemistry, 22.06.2019 07:30, avisconti571
Label a-f based on the table using c for concentrated and d for dilute
Answers: 2

Chemistry, 22.06.2019 12:00, daytonalive83481
Ican determine the molar mass of an element by looking on the under the atomic mass for the element. for example the molar mass of phosphorus is 30.974 grams/mole. avogadro’s number tells me the amount of representative particles in 1 mole of any substance. this means 12.011 gram sample of carbon and a 32.0 gram sample of sulfur have the same number of atoms.
Answers: 1
You know the right answer?
If the formula of ammonium nitrate is NH4NO3, determine how many moles of the solid were dissolved i...
Questions in other subjects:


Mathematics, 05.11.2020 01:00


English, 05.11.2020 01:00

English, 05.11.2020 01:00

English, 05.11.2020 01:00

Computers and Technology, 05.11.2020 01:00

Chemistry, 05.11.2020 01:00


Mathematics, 05.11.2020 01:00



