2 H202 - 2 H2O + O2

Chemistry, 22.04.2021 04:40 cjasmine626
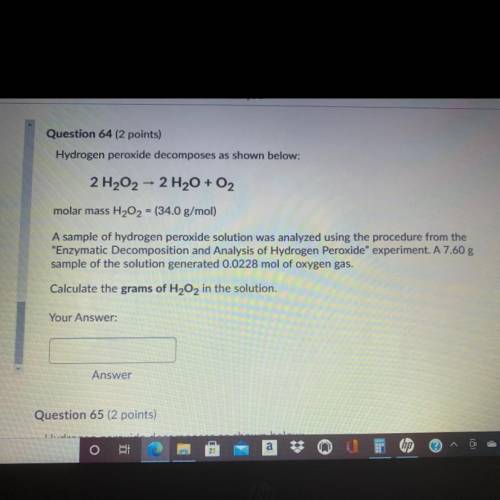
Question 64 (2 points)
Hydrogen peroxide decomposes as shown below:
2 H202 - 2 H2O + O2
molar mass H202 = (34.0 g/mol)
A sample of hydrogen peroxide solution was analyzed using the procedure from the
"Enzymatic Decomposition and Analysis of Hydrogen peroxide" experiment. A 7.60 g
sample of the solution generated 0.0228 mol of oxygen gas.
Calculate the grams of H2O2 in the solution.
Your
Answer

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:30, melikefood01
Sally is making a model of a magnesium atom with an atomic mass number of 24 for her chemistry class. she has foam balls for the protons, neutrons, and electrons. she has added 6 neutrons to her model so far. how many more neutrons does she need to add to complete her neutral atom of magnesium?
Answers: 1

Chemistry, 22.06.2019 12:00, vannitling12p4w44f
What is the percentage of hydrogen in nitrogen trihydride
Answers: 1

Chemistry, 22.06.2019 12:40, carebear60
Quiz1. which physical state of nitrogen has the highest entropy? a solid© b gasoc liquid
Answers: 1

Chemistry, 22.06.2019 14:00, leahstubbs
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3
You know the right answer?
Question 64 (2 points)
Hydrogen peroxide decomposes as shown below:
2 H202 - 2 H2O + O2
2 H202 - 2 H2O + O2
Questions in other subjects:


History, 14.02.2021 06:20

Mathematics, 14.02.2021 06:20

Mathematics, 14.02.2021 06:20


Mathematics, 14.02.2021 06:20

Mathematics, 14.02.2021 06:20

English, 14.02.2021 06:20


History, 14.02.2021 06:20



