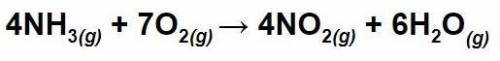Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:00, Sarahinator04
0.09 moles of sodium sulfate in 12 ml of solution
Answers: 3

Chemistry, 23.06.2019 03:30, damyonfenton13
If 2 molecules of one reactant combine with 3 molecules of another to produce 5 molecules of a product, then what is the representation of the reaction?
Answers: 1

Chemistry, 23.06.2019 06:50, isabellainksow87vn
The student repeated the experiment using a higher concentration of acid. the same volume of acid and the same mass of magnesium ribbon were used. what volume of hydrogen gas would have been produced after 60 seconds?
Answers: 1
You know the right answer?
How many grams of ammonia will be required to produce 12.0g of water given the balanced chemical equ...
Questions in other subjects:

Mathematics, 10.12.2020 01:40





Health, 10.12.2020 01:40


Physics, 10.12.2020 01:40

Computers and Technology, 10.12.2020 01:40