
Chemistry, 22.04.2021 01:00 mivantechenko9751
A solution is prepared by dissolving 0.23 mol of chloroacetic acid and 0.27 mol of sodium chloroacetate in water sufficient to yield 1.00 L of solution. The addition of 0.05 mol of HCl to this buffer solution causes the pH to drop slightly. The pH does not decrease drastically because the HCl reacts with the _ present in the buffer solution. The 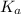 of chloroacetic acid is 1.36 x 10⁻³.
a. H₂O
of chloroacetic acid is 1.36 x 10⁻³.
a. H₂O
b. Na⁺
c. chloroacetate ion
d. chloroacetic acid
e. This is a buffer solution: the pH does not change upon addition of acid or base.

Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 22:30, brianna5626
How do limiting factors most affect population size? ostop population growthrestrict population growthincrease population sizeresult in positive impactso
Answers: 1

Chemistry, 22.06.2019 23:30, sanociahnoel
The density of the solid phase of a substance is 0.90 g/cm3 and the density of the liquid phase is 1.0 g/cm3. a large increase in pressure will a. lower the freezing point b. raise the freezing point c. lower the boiling point d. raise the triple point e. lower the triple point
Answers: 1

Chemistry, 23.06.2019 10:20, NasirKA7372
What do many bases have in common? apex chemistry sem 2
Answers: 2
You know the right answer?
A solution is prepared by dissolving 0.23 mol of chloroacetic acid and 0.27 mol of sodium chloroacet...
Questions in other subjects:

English, 02.09.2019 10:50


Business, 02.09.2019 10:50


Mathematics, 02.09.2019 10:50


Chemistry, 02.09.2019 10:50

Social Studies, 02.09.2019 10:50

Business, 02.09.2019 10:50

History, 02.09.2019 10:50



