
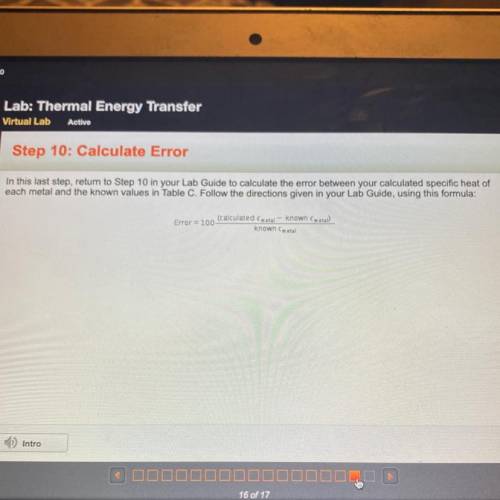
In this last step, return to Step 10 in your Lab Guide to calculate the error between your calculated specific heat of
each metal and the known values in Table C. Follow the directions given in your Lab Guide, using this formula:
(calculated metal - known (metal)
Error = 100
known Cmetal

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 16:00, bbrogle5154
If 15 drops of ethanol from a medical dropper weight 0.60g, how many drops does it takes from a dropper to dispense 1.0ml of ethanol? the density of ethanol is 0.80g/ml
Answers: 1

Chemistry, 22.06.2019 20:00, AaronEarlMerringer
What is the molar mass of the anhydrous compound? answer using four significant figures. 36.02 g/mol 120.15 g/mol 156.12 g/mol
Answers: 1

Chemistry, 22.06.2019 21:30, sierradanielle9280
In science class richard learns that a substance has a boiling point of 230 fahrenheit his teacher ask him to convert this temperature to degrees celsius what is the boiling point of his substance in degrees celsius
Answers: 3

Chemistry, 23.06.2019 05:30, khaylaperry
What is the morality of 2.50 l of solution that contains 1.85 mol of anhydrous sodium tetraborate?
Answers: 1
You know the right answer?
In this last step, return to Step 10 in your Lab Guide to calculate the error between your calculate...
Questions in other subjects:


Arts, 26.10.2020 05:20


Mathematics, 26.10.2020 05:20



Mathematics, 26.10.2020 05:20






