Answers: 3


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 22:00, jlegrand9098
4.25g sample of solid ammonium nitrate dissolves in 60.0g of water in a coffee-cup calorimeter, the temperature drops from 22.0 c to 16.9 c. assume that the specific heat of the solution is the same as that of pure water. calculate delta(h) (in kj/mol nh4no3) for the solution proces.
Answers: 2

Chemistry, 22.06.2019 22:30, lori90
Essay-alternative energy sources research sources of energy that are being developed. write a report of 350-400 words discussing the information you learned concerning the development of various energy sources and the impact that you think they will have on your life. include sources cited at the end of your report using the mla format. follow the rubric guidelines. note that wikipedia is not an appropriate resource for a research paper. worth 99
Answers: 3

Chemistry, 22.06.2019 23:30, Xavier8247
Rank the following four acids in order of increasing bronsted acidity : h2f+ , ch3oh, (ch3)2oh+ , ch3sh2+
Answers: 3
You know the right answer?
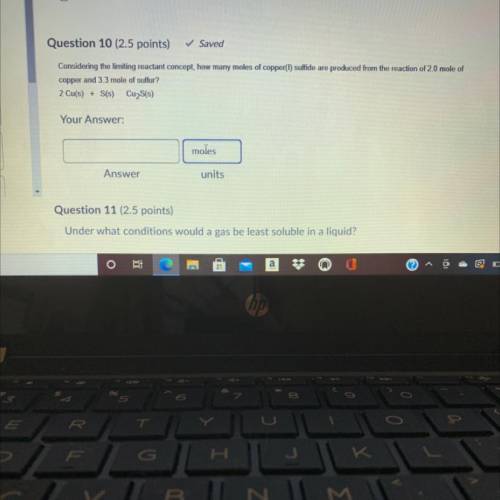
Considering the limiting reactant concept, how many moles of copper(t) sulfide are produced from the...
Questions in other subjects:


Mathematics, 20.10.2019 05:30

English, 20.10.2019 05:30


Advanced Placement (AP), 20.10.2019 05:30

Computers and Technology, 20.10.2019 05:30


Physics, 20.10.2019 05:30


History, 20.10.2019 05:30