
Chemistry, 20.04.2021 09:30 zahnjoey4661
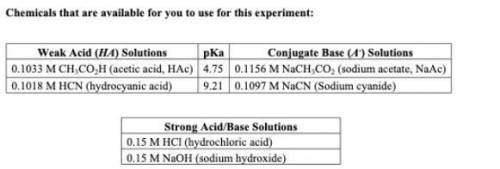
1. From the chemicals listed on your lab handout, write down the weak acid (with its pKa) and its conjugate base that would create a buffer that best fits your protein. Would you expect for your buffer to have more acid or more base?
My assigned protein is Xylanase and has an optimum pH of 5.5.
2. Buffers are used to the inhibit the change of pH upon the addition of strong acids and bases. If you were to add 0.1 M HCl to your buffer, would you expect the pH to change? If so, would the pH increase or decrease? What would happen if 0.1M NaOH were to be added instead?
3. Keeping your buffer composition from question 1 in mind, would you expect to use a larger volume of HCl or NaOH to change the pH of the buffer solution by one unit? Explain.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:00, lydiadmanautou04
Write a hypothesis that answers the lesson question, “while observing a chemical reaction, how can you tell which reactant is limiting? ” hypothesis: if a substance is the limiting reactant, then . . because . .
Answers: 1

Chemistry, 22.06.2019 04:20, lex68259100
Which formula can be used to calculate the molar mass of ammonia (nh3)? molar mass of n + molar mass of h 3 × molar mass of n + molar mass of h molar mass of n + 3 × molar mass of h 3 × molar mass of n + 3 × molar mass of h
Answers: 1

You know the right answer?
1. From the chemicals listed on your lab handout, write down the weak acid (with its pKa) and its co...
Questions in other subjects:

Mathematics, 22.10.2019 17:00





History, 22.10.2019 17:00


Social Studies, 22.10.2019 17:00


History, 22.10.2019 17:00




