Fe2o3 + h2 --> fe + h2o
a) what mass of hydrogen gas must be consumed to produe 10.0 g of...

Chemistry, 20.10.2019 14:00 yesharabaskoro
Fe2o3 + h2 --> fe + h2o
a) what mass of hydrogen gas must be consumed to produe 10.0 g of iron metal?
b) what mass of iron(lll) oxide, fe2o3, must be consumed to prepare 2.50g of iron metal

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:00, officialgraciela67
What is the volume occupied by 10.0 dm3 of gas at standard pressure after it has been compressedat constant temputure to 500.0 kpa?
Answers: 1

Chemistry, 22.06.2019 06:20, stephliu721
What is a property of a double replacement reaction
Answers: 1

Chemistry, 22.06.2019 10:40, rntaran2002
What is the ph of a 0.0010 m hno3? 1.0 3.0 4.0 5.0
Answers: 2

Chemistry, 22.06.2019 23:00, poolwaterisgross
How does the value of the equilibrium constant show that a reaction reaches equilibrium very quickly? (a) the equilibrium constant is large. (b) the equilibrium constant is small. (c) the equilibrium constant is zero. (d) the value of the equilibrium constant does not show how quickly a reaction comes to equilibrium.
Answers: 1
You know the right answer?
Questions in other subjects:

Arts, 05.02.2021 20:40

Mathematics, 05.02.2021 20:40


Mathematics, 05.02.2021 20:40


Mathematics, 05.02.2021 20:40


Biology, 05.02.2021 20:40

Mathematics, 05.02.2021 20:40

Mathematics, 05.02.2021 20:40

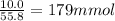 of Iron.
of Iron.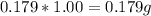 of dihydrogen.
of dihydrogen.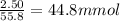 of Iron, hence we'll need
of Iron, hence we'll need 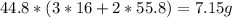 of Fe2O3.
of Fe2O3.

