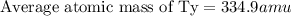Chemistry, 19.09.2019 08:00 loveniasummer71
Tyserium (ty) consists of two isotopes. one isotope has a mass of 331 amu and is 35.0 % abundant. the other isotope is 337 amu and is 65.0 % abundant. what is the mass of ty as it appears on the periodic table?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, granthazenp5e9mj
Which feature do highland climates have that lower elevation areas do not?
Answers: 1


Chemistry, 22.06.2019 10:30, angemango3423
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1

Chemistry, 22.06.2019 14:30, malenacastillo4887
For the reaction shown, find the limiting reactant for each of the following initial amounts of reactants. 4al(s)+3o2(g)→2al2o3(s) a) 1 molal, 1 mol o2 b) 4 molal, 2.6 mol o2 c) 16 molal, 13 mol o2 d) 7.4 molal, 6.5 mol o2
Answers: 3
You know the right answer?
Tyserium (ty) consists of two isotopes. one isotope has a mass of 331 amu and is 35.0 % abundant. th...
Questions in other subjects:


Spanish, 13.07.2019 19:00

Mathematics, 13.07.2019 19:00



Mathematics, 13.07.2019 19:00

Mathematics, 13.07.2019 19:00



Mathematics, 13.07.2019 19:00

 .....(1)
.....(1)![\text{Average atomic mass of Ty}=[(331\times 0.35)+(337\times 0.65)]](/tpl/images/0242/4851/befca.png)