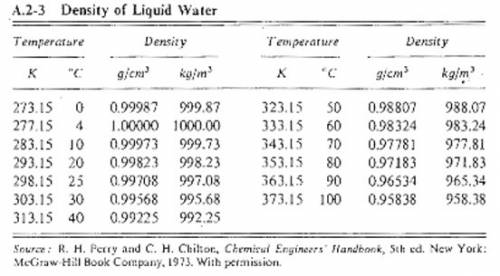
Suppose you were calibrating a 100.0 ml volumetric flask using distilled water. the flask temperature was at 20°c, and you assumed that the distilled water was as well. however, you later discover that the actual water temperature was 11°c instead. how is the mass of the 100.0 ml of distilled water you measured at 11°c different from the mass of 100.0 ml of distilled water at 20°c?

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 17:00, emma3216
In a heat engine of 1000 j of heat enters the system and the piston does 500 j of work what is the final internal energy of the system if the inital energy was 2000 j we have to do all of these down here 1)write the equation 2)list out your know variables 3)plug the numbers into the equations 4)solve 5)write your solution statemtn that includes inital energuy and final energuy added
Answers: 1

You know the right answer?
Suppose you were calibrating a 100.0 ml volumetric flask using distilled water. the flask temperatur...
Questions in other subjects:





Mathematics, 30.03.2021 16:50

Health, 30.03.2021 16:50

Biology, 30.03.2021 16:50



Biology, 30.03.2021 16:50