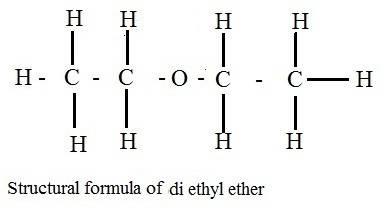Chemistry, 05.10.2019 20:00 mallardmya2006
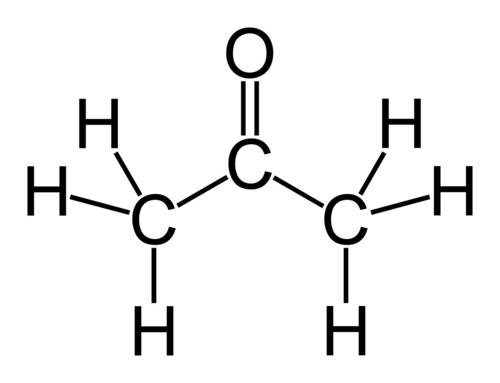
Why does ch3coch3 has stronger intermolecular forces than c2h5oc2h5? even though both have dipole-dipole as their imf, but c2h5oc2h5 has a larger molecular weight and as the molecular weight increases, the imf get stronger. so why it is the opposite here?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:00, officialgraciela67
What is the volume occupied by 10.0 dm3 of gas at standard pressure after it has been compressedat constant temputure to 500.0 kpa?
Answers: 1

Chemistry, 22.06.2019 10:30, kdenormandie3122
Geothermal energy for industrial use is available almost anywhere. a. true b. false
Answers: 2

Chemistry, 22.06.2019 20:30, demarcuswiseman
From the choices provided below, list the reagent(s) in order that will react with cyclopentanone to form the compound shown below.
Answers: 2
You know the right answer?
Why does ch3coch3 has stronger intermolecular forces than c2h5oc2h5? even though both have dipole-d...
Questions in other subjects:

Mathematics, 22.10.2020 19:01

Mathematics, 22.10.2020 19:01


Business, 22.10.2020 19:01

Mathematics, 22.10.2020 19:01



History, 22.10.2020 19:01


Social Studies, 22.10.2020 19:01