Chemistry, 19.12.2019 13:31 dakshshberry
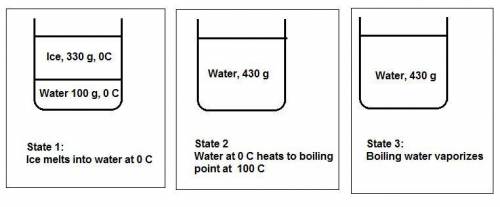
Water's heat of fusion is 80. cal/g , its specific heat is 1.0calg⋅∘c, and its heat of vaporization is 540 cal/g . a canister is filled with 330 g of ice and 100. g of liquid water, both at 0 ∘c . the canister is placed in an oven until all the h2o has boiled off and the canister is empty. how much energy in calories was absorbed?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:00, brapmaster764
What is the formula that this ionic compounds could form sr2+p3-o2-
Answers: 3


Chemistry, 22.06.2019 12:00, BreBreDoeCCx
Ageochemist examines a piece of metal that he found in the soil. he performs tests to identify the metal from its density, electrical conductivity, and melting point. which statement best describes his investigation? a. he is determining physical properties that are sufficient to identify the metal. b. he is determining chemical properties that are sufficient to identify the metal. c. he is determining physical properties that are insufficient to identify the metal. d. he is determining chemical properties that are insufficient to identify the metal.
Answers: 3

Chemistry, 23.06.2019 02:00, Paytonsmommy09
Butane gas reacts with oxygen gas to give carbon dioxide gas and water vapor (gas). if you mix butane and oxygen in the correct stoichiometric ratio, and if the total pressure of the mixture is 390 mmhg, what is the pressure (in mmhg) of water vapor after the reaction is completed (temperature and volume do not change).
Answers: 2
You know the right answer?
Water's heat of fusion is 80. cal/g , its specific heat is 1.0calg⋅∘c, and its heat of vaporization...
Questions in other subjects:

Mathematics, 26.06.2019 03:00

English, 26.06.2019 03:00


Biology, 26.06.2019 03:00

Advanced Placement (AP), 26.06.2019 03:00

Mathematics, 26.06.2019 03:00



Social Studies, 26.06.2019 03:00