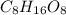Chemistry, 25.09.2019 15:00 liferoblox666
Acompound is 40.0% c, 6.70% h, and 53.3% o by mass. assume that we have a 100.-g sample of this compound. the molecular formula mass of this compound is 240 amu . what are the subscripts in the actual molecular formula?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:00, lakenyagillard79
Acurium-245 nucleus is hit with a neutron and changes as shown by the equation. complete the equation by filling in the missing parts. 52
Answers: 2



Chemistry, 22.06.2019 22:30, jkjjoijjm5928
Akno3 solution containing 51 g of kno3 per 100.0 g of water is cooled from 40 ∘c to 0 ∘c. what will happen during cooling?
Answers: 3
You know the right answer?
Acompound is 40.0% c, 6.70% h, and 53.3% o by mass. assume that we have a 100.-g sample of this comp...
Questions in other subjects:

Mathematics, 02.11.2020 19:50



Chemistry, 02.11.2020 19:50

Mathematics, 02.11.2020 19:50

Mathematics, 02.11.2020 19:50



Arts, 02.11.2020 19:50

English, 02.11.2020 19:50