Chemistry, 16.09.2019 10:30 asseatingbandit
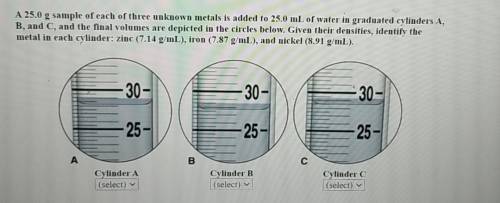
44 a 25.0-g sample of each of three unknown metals is added to 25.0 ml of water in graduated cylinders a, b, and c, and the final volumes are depicted in the circles below. given their densities, identify the metal in each cylinder: zinc (7.14 g/ml), iron (7.87 g/ml), or nickel (8.91 g/ml).

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 14:00, ashlynneboogs0056
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3

Chemistry, 23.06.2019 09:00, alyssa0888
Water is a highly important natural resource. which of these would be the best method to conserve water? a) drinking bottled water b) monitoring the ph of rivers c) treating and re-using wastewater d) testing nitrate levels in groundwater
Answers: 1

Chemistry, 23.06.2019 10:30, piratesfc02
When a wire with a current is placed in a magnetic field, electrical energy is transformed into mechanical energy select the best answer from the choices provided t f
Answers: 2
You know the right answer?
44 a 25.0-g sample of each of three unknown metals is added to 25.0 ml of water in graduated cylinde...
Questions in other subjects:

History, 08.12.2020 04:30


Biology, 08.12.2020 04:30

Mathematics, 08.12.2020 04:30


English, 08.12.2020 04:30


Biology, 08.12.2020 04:30


Mathematics, 08.12.2020 04:30