
Chemistry, 24.12.2019 06:31 rebeccacruzz2017
The graph shows the reaction pathway for the reaction q + r n + m. interpret the graph by describing what each of the letters (a through g) represents and by explaining whether the reaction is endothermic or exothermic and how you know.
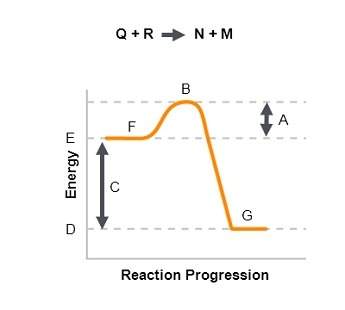

Answers: 3


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 14:30, Cartucho1978
According to le chatelier’s principle, a system in chemical equilibrium responds to stress by shifting the equilibrium in a direction that reduces the stress. normalizes the stress. increases the stress. changes the stress.
Answers: 1

Chemistry, 22.06.2019 18:50, emily9656
Which of the following is a conclusion that resulted from ernest rutherford’s scattering experiment? (will mark brainliest) a. the nucleus is negatively charged b. the atom is a dense solid and is indivisible c. the mass is conserved when atoms react chemically d. the nucleus is very small and the atom is mostly empty space
Answers: 3

Chemistry, 22.06.2019 19:00, miguel454545
Avolleyball player hit a ball with a mass of 0.25 kg. the average acceleration of the ball is 15.5 m/s². how much force did the volleyball player apply to the ball? 62.0 n 3.87 n 62.0 m/s² 3.87 m/s²
Answers: 2
You know the right answer?
The graph shows the reaction pathway for the reaction q + r n + m. interpret the graph by describing...
Questions in other subjects:

Mathematics, 12.09.2019 23:30

Mathematics, 12.09.2019 23:30










