
Chemistry, 24.08.2019 23:20 eeromaki1321
For the reaction:
6li(s)+n2(g)→2li3n(s)
determine:
the mass of n2 needed to react with 0.536 moles of li.
the number of moles of li required to make 46.4 g of li3n.
the mass in grams of li3n produced from 3.65 g li.
the number of moles of lithium needed to react with 7.00 grams of n2.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:40, wiltseliz4800
What does the process of natural selection involve
Answers: 1

Chemistry, 22.06.2019 09:00, Aminton737
Plz mark brainliest 30 points1) find the momentum of a 12 kg snowball that is rolling with a velocity of 9 m/s.2) an 8 ball with a mass of .5 kg is sitting at rest. it is hit by the cue ball (1 kg) traveling at 2.5 m/s. if the cue ball is at rest after the collision, how fast is the 8 ball traveling after the collision? 3) two football players are running toward each other. if the offensive player is 75 kg and is running 8 m/s, how fast must the 60 kg defensive player run in order for the two players to hit and stop?
Answers: 1

Chemistry, 22.06.2019 12:30, ethanw8973
If 22.5 liters of oxygen reacted with excess of hydrogen, how many liters of water vapor could be produced?
Answers: 3

Chemistry, 22.06.2019 21:00, estherdinhllama
Read "who built the pyramids? ”. leave this link open while you answer the questions throughout the assignment. give at least two reasons why some people claim the pyramids of giza were constructed by aliens.
Answers: 1
You know the right answer?
For the reaction:
6li(s)+n2(g)→2li3n(s)
determine:
the mass of n2 needed to reac...
6li(s)+n2(g)→2li3n(s)
determine:
the mass of n2 needed to reac...
Questions in other subjects:

Mathematics, 21.04.2020 21:27






English, 21.04.2020 21:27

Mathematics, 21.04.2020 21:27


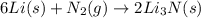
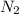 needed to react with 0.536 moles of Li.
needed to react with 0.536 moles of Li.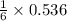 moles of
moles of ![N_2[tex] gas needed:[tex]=28 g/mol\times 0.0893 mol=2.5004 g](/tpl/images/0194/9373/6ce3e.png)
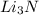
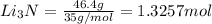
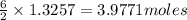

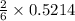 that is 0.1738 moles of
that is 0.1738 moles of 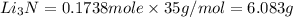
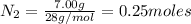
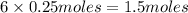 of lithium
of lithium

