
Chemistry, 16.01.2020 23:31 PeachyPeaches1489
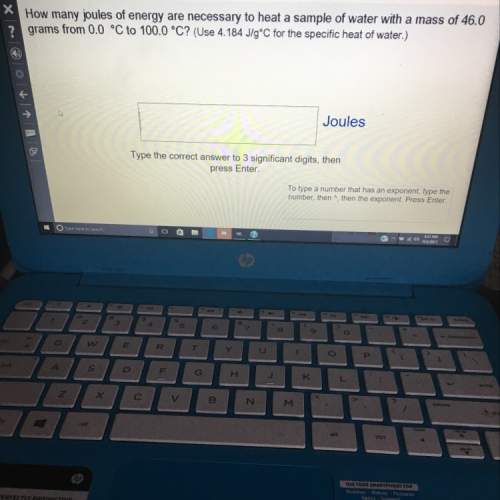
How many joules of energy are necessary to heat a sample of water with a mass of 46.0 grams from 0.0 celsius to 100.0 celsius? (use 4.184 j/g celsius for the specific heat of water.)
type the correct answer to 3 significant digits. if the answer has an exponent, type the number, then ^, then the exponent.
(the question is on the picture.)

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:40, yah2muchh
Calculate: select the worksheet tab. this tab you calculate the analyte concentration. fill in the first set of boxes ("moles h2so4" and "moles naoh") based on the coefficients in the balanced equation. (if there is no coefficient, the value is 1.) record the appropriate volumes in the "ml naoh" and "ml h2so4" boxes. record the concentration of the titrant in the m naoh box. click calculate. what is the concentration listed
Answers: 2

Chemistry, 22.06.2019 12:00, WinterStrikesBack
Solutions of sodium carbonate and silver nitrate react to form solid silver carbonate and a solution of sodium nitrate. a solution containing 3.50 g of sodium carbonate is mixed with one containing 5.00 g of silver nitrate. how many grams of sodium carbonate, silver nitrate, silver carbonate, and sodium nitrate are present after the reaction is complete?
Answers: 2

Chemistry, 22.06.2019 12:30, AlexRavenwood127
What metric units would you use to measure the thickness of a key
Answers: 3

Chemistry, 22.06.2019 16:00, bbrogle5154
If 15 drops of ethanol from a medical dropper weight 0.60g, how many drops does it takes from a dropper to dispense 1.0ml of ethanol? the density of ethanol is 0.80g/ml
Answers: 1
You know the right answer?
How many joules of energy are necessary to heat a sample of water with a mass of 46.0 grams from 0.0...
Questions in other subjects:



Biology, 04.08.2019 15:30

History, 04.08.2019 15:30

Spanish, 04.08.2019 15:30

Mathematics, 04.08.2019 15:30



Chemistry, 04.08.2019 15:30



