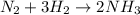Chemistry, 31.08.2019 16:30 icashaypabozp
In 1912, chemist fritz haber developed a process that combined nitrogen from the air with hydrogen at high temperatures and pressures to make ammonia. specifically, the process involved combining one molecule of nitrogen gas (n2) with three molecules of hydrogen gas (h2) to get two molecules of ammonia (nh3). if you write this process in a symbol format, it looks like this:
n2 + 3h2 → 2nh3
explain whether this is a chemical or physical change, and why. does it involve elements, compounds, mixtures, or pure substances? also describe how many atoms are involved before and after. what do you notice about the number of atoms?

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 21.06.2019 23:10, ChloeLiz7111
Nitrogen (n), phosphorus (p), and potassium (k) are the main nutrients in plant fertilizers. according to an industry convention, the numbers on the label refer to the mass percents of n, p2o5, and k2o, in that order. calculate the n: p: k ratio of a 30: 10: 10 fertilizer in terms of moles of each element, and express it as x: y: 1.0.
Answers: 1

Chemistry, 22.06.2019 16:00, annsmith66
What statement goes against the kinetic theory of gases
Answers: 1

You know the right answer?
In 1912, chemist fritz haber developed a process that combined nitrogen from the air with hydrogen a...
Questions in other subjects:






English, 17.09.2019 12:30

Mathematics, 17.09.2019 12:30

Biology, 17.09.2019 12:30

Physics, 17.09.2019 12:30